"Eligibility Criteria" for TGA Priority Review Pathway
The fast track Provisional Registration pathway which all COVID-19 vaccines and remdesivir passed through in Australia required no alternative treatment could be available - Archives vs Now.
This will not be a big write up as I don’t have the time, but I want to make sure I capture this piece of information should anyone find it useful for education or litigation purposes!
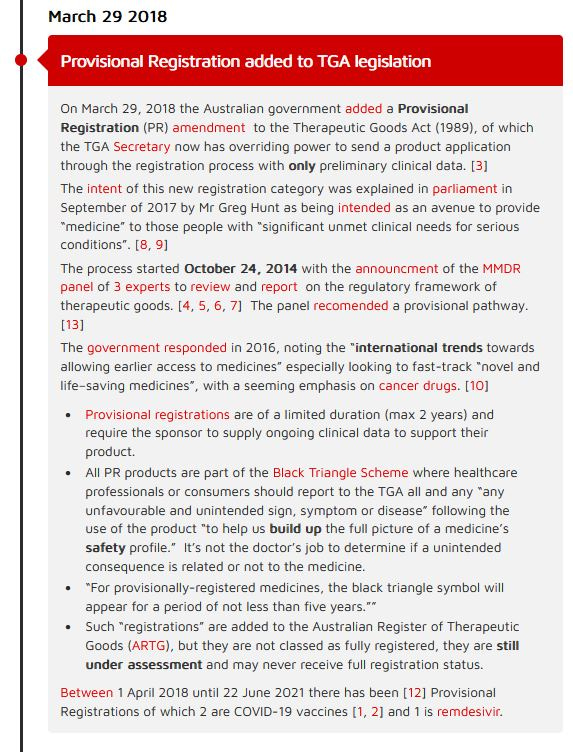
A Provisional Registration clause was added to the Therapeutic Goods Act on March 29, 2018, which allowed for Priority Review for any product that met certain criteria.
Provisional Registration was one of the first data points I added to the COVID-19 pandemic timeline, you can read more about the history for this specific data point on my website HERE (or click image), with links to all sources.
I knew that in the United States that Emergency Use Authorisation would not be provided for a new product if there was an already registered effective alternative treatment. That is why there was a war on ivermectin, Vitamin C, hydroxychloroquine and any other product that threatened the objective of getting a new technology mRNA “vaccine” platform into the regulatory systems around the world.
Did Australia have the requirement for no “alternative” product available?
Ever since mid 2020, I have been looking out for the equivalent document here in Australia that states no other treatment options can be available for a vaccine to be considered for the fast-track pathway, as it was obvious to me the system didn’t want an alternative “cure”.
It was on March 27, 2020 that the TGA published their page “No evidence to support intravenous high-dose vitamin C in the management of COVID-19”, it was then I suspected something was up. The very first paper they referenced actually showed benefit for sepsis - even though JAMA insisted the paper had to be written up as a “negative” outcome if it was to be published! Did the advisory body not read the paper?
So to my point. For a product to be considered eligible to enter the fast track regulatory pathway it had to meet certain criteria, and one of those was that no alternative treatment could be available.
TGA: Priority review designation eligibility criteria (Archive)
From 2017 I found the archive document as the Provisional Registration was being worked into legislation. One of the criteria was there had to be:
2(i) no therapeutic goods that are intended to treat, prevent or diagnose the condition are included in the Register.
I don’t think the “vaccines” could have met point 2(ii), so [they] needed to kill all alternative treatments.

You can read the archived documents for yourself:
Priority review designation eligibility criteria - HERE
A step-by step guide for prescription medicines (which for TGA includes vaccines) - HERE
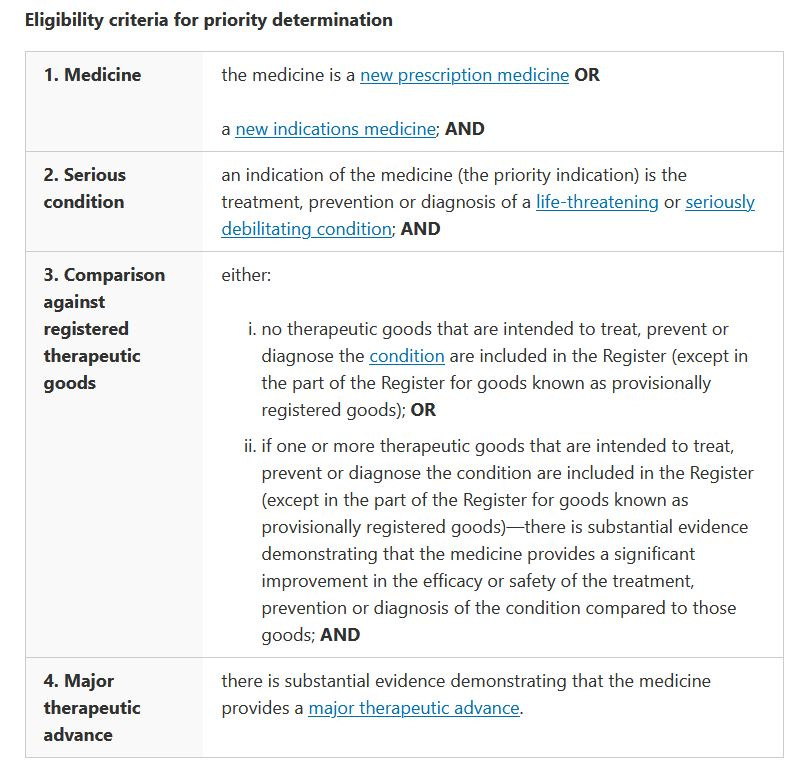
Priority determination eligibility criteria (2023)
Today, the TGA has included an additional criteria for determination
Step 1, starts with “the medicine is a new prescription medicine OR a new indications medicine” - READ
I suspect this has been added to allow any new mRNA “vaccine” product to enter the fast track Provisional pathway - same “biological” but with a new “indication” i.e. a new target disease ready for the next plan-demic.
Why would “prevention” be included unless vaccines were already intened to be fast tracked!
In the July 26, 2017 TGA published their web page announcing it’s intention to impement “a Priority review pathway for the registration of novel prescription medicines for Australian patients.”
Now is someone who is not sick a “patient”?
It also stated
The eligibility criteria are designed to ensure that only medicines providing the most benefit to patients are eligible.
Imagine if Australia didn’t have the fast track pathway in time for pandemic 2020! What would be the benefit to the Australian people for “aligning” with other regulators?
Maybe I am wrong with the exact date that Provisional Registration began in Australia. Maybe it wasn’t March 29, 2018, but actually went into effect 5 months earlier on July 26, 2017 when TGA began advertising it on their website. I originally searched through the legislations to see when the new clause it was added, I may have missed an earlier update. Though it does say “pending passage of required changes to the regulations” on the TGA page, yet priority designation was included in the fees schedule in July 2017. I’m going to have to let this rest here for the moment.
Support Jack’s Work
If you find the content at Totality of Evidence useful for yourself or to help awaken your family and friends, please consider becoming a paid subscriber so I can continue to add to this historical record.
From time to time I’ll add a stack here, but most of my work is done on the website, your support here, supports my work there!
Otherwise share the website on social media and subscribe to my substack so you get my next stack in your inbox!







This makes sense in the context of how it all happened. The path to remdesivir required a crude defeat of HCQ, but not dominating evidence of its own success.
The whole plan had to satisfy laws of multiple nations, but there was sculpting of that process along the way.
@julesonthebeach.substack.com/