FDA admits "vaccines" do not have to prevent infection or tranmission
So if they just "prevent" symptoms, doesn't that make these mRNA "vaccines" a drug?
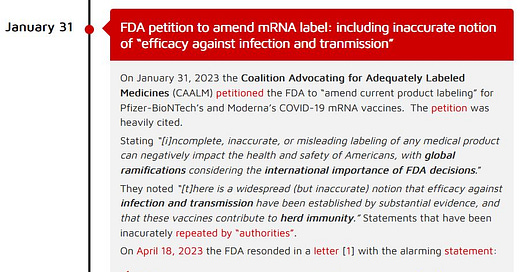
Thanks to the Coalition Advocating for Adequately Labeled Medicines (CAALM) who petitioned the FDA to “amend current product labeling” for Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines, we now have in writing that the FDA does not require a product that the manufacture refers to as a “vaccine” to actually have a prophylactic (prevent infection) or community (prevent transmission) effect.
FDA’s SHOCKING admission (bold is mine) - see page 11
It is important to note that FDA’s authorization and licensure standards for vaccines do not require demonstration of the prevention of infection or transmission. A vaccine can meet the licensure standard if the vaccine’s benefits of protecting against disease [SYMPTOMS] outweigh the vaccine’s risks for the licensed use. There is no requirement that the vaccine also prevents infection with the pathogen that can cause the disease or transmission of that pathogen to others. Similarly, a vaccine can meet the EUA standard without any evidence that the vaccine prevents infection or transmission. To that end, there is no requirement that the clinical trials supporting a vaccine’s licensure or authorization be designed to determine whether the vaccine prevents infection of a pathogen or transmission of that pathogen to others.
So in essence any product can be labelled a vaccine if it reduces symptoms aka the dis-ease - ok..so long as it stimulates antibodies! (But not all antibodies are created equal).
Definition of a vaccine
The definition of a vaccine changed on September 1, 2021 - to any product that “stimulates the body’s immune response”, it used to require “immunity”.
Nomenclature matters
FDA added a caveat to reinforce that “No FDA licensed or authorized vaccine is 100% effective in preventing disease”. Note they use “disease” and not “pathogen”. Nomenclature matters!
They don’t even have to look
Remember in the reply to CAALM the FDA stated the clinical trials don’t even need to be designed to look at transmission or infection!
…there is no requirement that the clinical trials supporting a vaccine’s licensure or authorization be designed to determine whether the vaccine prevents infection of a pathogen or transmission of that pathogen to others.
“Preventing” or reducing symptoms - isn’t that the job of a drug?
If the product is targeted to reduce symptoms isn’t it more logical for such a product to be called a “drug” and as such go through that a drug regulatory pipeline, and keep “vaccines” for products that target a prophylactic benefit?
FDA’s decisions has a global impact
The petition made an important point for all of us outside of the USA - the “global ramifications” of all FDA decisions (and CDC for that matter).
CAALM stated:
“[i]ncomplete, inaccurate, or misleading labeling of any medical product can negatively impact the health and safety of Americans, with global ramifications considering the international importance of FDA decisions.”
Pandemic Timeline
I’ve added these two datapoints to the Pandemic Timeline as they are important to capture.
January 31, 2023 - CAALM petition - HERE
April 18, 2023 - FDA’s reply - HERE
Note: this is but one point extracted from the CAALM petition and the FDA’s response - it’s worth the read.
Help support my work
If you find this article but more importantly the content on my website Totality of Evidence useful, please share, subscribe and consider becoming a paid subscriber. Thanks to those who have contributed to date - I am very grateful.








Great material. It will take time to go through it.
So in the world of logic yes they are a drug unfortunately that doesn't seem to be the world these clowns live in