What is the legal definition of "a vaccine" in Australia?
The Therapeutics Goods Act and Regulations do not define "vaccine", yet they stipulate the need for an Advisory Committee on Vaccines (ACV). So what allows a product to be classified as a vaccine?
Before 2021 I had never looked at our government legislations, sadly I was ignorant to how the legislative system worked, but I was lucky to have a “legal mind” show me the basics of how the Acts and Regulations were set out. Although I am no expert in interpreting these documents, I can read, and cross reference and do word searches.
With regards to Australia’s medicines regulator, the Therapeutic Goods Administration (TGA), I have been stunned to find there is no legal definition written into either the Therapeutic Goods Act or the Regulations, for what legally constitutes a product to be classified as a “vaccine”. A vaccine regulatory pathway is very different to a drug/medicine pathway, it is not as stringent. Genotoxicity and other toxicity assessments were bypassed. So that begs the question, under what legal criteria is a product allowed to enter the regulatory process in Australia under the designation of it being called a “vaccine”?
We can all have opinions about what a vaccine is, and the TGA has a web page with statements of what is a vaccine (see below), but they are not definitions, and from what I can see the loose statements they provide are not a legal definition of a vaccine.
My question is, what criteria legally allows a medical product to be determined to be a vaccine, and be regulated as such?
My search for the definition of a “Vaccine”
Let me walk you through my thought process and show you the documents I’ve searched through to try to find an answer, so you can check for yourself.
Australia’s drug regulator , is the Therapeutics Goods Administration (TGA) as is part of the Health Products Regulation Group (HPRG) within the Department of Health and Ageing. It “was established in 1989 as the main Australian Government entity responsible for ensuring that medicines and medical devices used by Australian consumers are evaluated and regulated before they reach the market and monitored once they are in use.”1
The TGA is governed by a legislative “Act” ( Therapeutic Goods Act 1989 ) [ARCHIVE] and respective “Regulations” (Therapeutic Goods Regulations 1990) [ARCHIVE] which provides the rules for them to work within for assessing and approving medicines or medical devices. Once approved, the therapeutic products are placed on the Australian Therapeutic Goods Register (ARTG), and hence they are then referred to as Registered products. The TGA are responsible for regulating the supply, import, export, manufacturing, packaging and advertising of all therapeutic goods for Australia23
[I started to draft this article in late 2023, but I see now, as I review this article that the Australian Government have changed the format of the Federal Register of Legislation website. Here is a January 13, 2020 archived version of the Act, which you can compare to the current redirected format. I also provided [ARCHIVE] links above and below for this reason. ]
In the Act and Regulation you’ll find some definitions, but neither document contains a definition or “Interpretation” for what is deemed to be a “vaccine”, in fact the words vaccine(s) doesn’t even appear in The Act.
The Regulations define a “virucide” which “means a chemical agent that renders a virus non-infective”.
The Regulations define a “sterilant” which “means a chemical agent that kills microbes with the result that the sterility assurance level of a microbial survivor is less than 10‑6.”
They define “gene therapy” which “means the in vivo transfer of DNA or RNA into the cells of human recipients.” (Which happens to be the exact mode of action of the COVID-19 genetic LNP/modified mRNA products that were determined, via the provisional registration pathway, to be classified as a vaccine, and not a gene therapy)
The Regulations also define sporicide, new prescription medicine, mother tincture, hospital grade disinfectant, homoeopathic preparation, high level disinfectant, herbal substance, fungicide, antiseptic. But they DO NOT define a “vaccine”. Though curiously they don't define antibiotic either, of which it is only mentioned once in the Regulations document, unlike vaccine which is mentioned multiple times.
A “medical device” it is not defined in the regulations but has it's own regulations namely the Therapeutic Goods (Medical Devices) Regulations 2002.
The document defines “biological medicine” to include "a vaccine, a peptide, a protein or polysaccharide-based” which is “derived from a human, animal or other organism, or produced through recombinant technology or biotechnology; and [is] of a kind specified in item 1 of Part 1 of Schedule 10: or…”

See if you can make sense of point (iii) being item 1 of Part 1 of Schedule 10. These regulations are not straight forward to understand.
So is it enough for a product to be considered "a vaccine” if it is “produced through … biotechnology”?
So we’ve established that in Australia a “vaccine” is a biological medicine. The regulations also calls for the establishment of an Advisory Committee on Vaccines (ACV) to assess vaccines.
How can an advisory committee on a specific product type be called, when that product type has not been defined? On what basis is a product deemed to be a “vaccine” and appropriate for consideration by the ACV?
All these questions are especially important as a “vaccine” follows a different regulatory pathway to a “medicine”.
Other questions that I would like to find answers to include:
Who in the regulatory system determines whether a product can be categorised as a “vaccine”? I know it is the Secretary of the TGA that “determines” whether a product enters the provisional pathway, I guess at the same time they assess the product type, and thus which pathway it falls into.
What I sense is that the sponsor informs the TGA what pathway they’d like their product to be considered. If the TGA rejects that determination, they don’t get paid.
Is it the TGA Secretary that has the sole gatekeeper responsibility to decide if a product can go through the regulatory process as “a vaccine”? Are other entities involved in this decision?
If the secretary is solely responsible, then on what legal framework or parameters do they use to make their determination? Where is this written?
In short, is there a legal definition of a “vaccine”?
Why doesn’t the Therapeutic Goods Regulations NOT include the definition of a vaccine? Why was this left out? How can we get one added in.
These don’t have clear answers, and maybe a Freedom of Information request would be needed to get some answers.
Original TGA Regulations only defined “drug”
When I took a dive back into the original 6th December 1990 version of the Regulations, “drugs” was the only logical category that a vaccine could be allotted. From the definition (see image below) it seems vaccines don’t fit (a), and so they would have to fit (b), that being “any other therapeutic goods declared by the Secretary” of the TGA, which are not devices. It seems vaccines have simply been grandfathered into our regulatory system, without any clear and defining criteria of entry.

What does the TGA’ official website say about defining a vaccine!
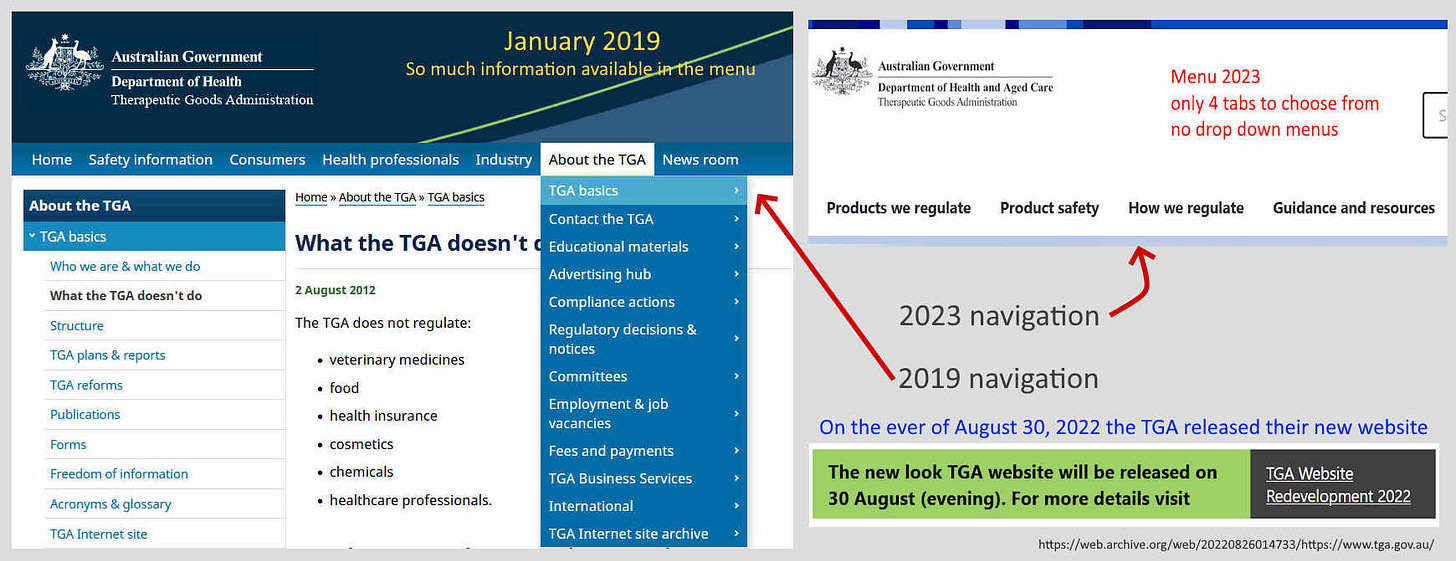
As an aside, it’s getting harder to find information on the TGA’s official website now that they have changed the design, which is allegedly to be inline with the Government Style Manual.
On the eve of August 30, 2022 the TGA released their new look website which was supposed to make it easier to navigate! I find this new version harder to locate information and their internal search tool is not user friendly. Compare the new site to their earlier version, such as 2019, which was set out with a logical search menu on the home page.
I just discover that in 2019 the TGA had an internet archive committee, though they appeared to have stopped updating their presentations on the web in 2017. TGA also state on their Website Redevelopment 2022 page that the old website content will be archived on the National Library of Australia web archive called TROVE. Good luck trying to navigate that beast!
The TGA’s stated reasoning for making the website changes" is":
“You told us you wanted the information on our new website to be current and correct. To reach this goal, we looked at our very old content and removed anything that was no longer relevant, accurate, or current.”
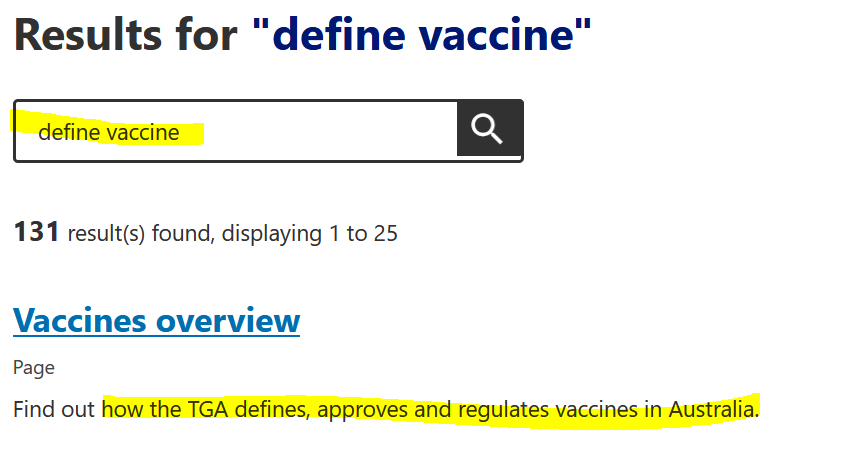
Well that is nice to know they have removed anything that is not “accurate”, there appears to be a lot missing!!! So this implies anything on their new website is now accurate. Lets use it now to search for what they currently deem to be a “vaccine”. You’re going to have to bare with me as this is not an easy task.
The Vaccine overview link leads to a November 14, 2023 page where there is no definition but loose, non-committal statements, or bold promises of protecting others. What ever “protection” means. If you’ve watched any of Aaron Siri’s presentations from his work with ICAN, you’ll know these statements are misleading generalisations at best.
What the heck does “protect” mean. Vitamin C can protect me form getting sick from the flu, so does that make vitamin C a vaccine? It seems a vaccine doesn’t have to contain any of the listed ingredients. What about homeopathic remedies that are potentised based on a bacteria or viral fluid, could they be considered a vaccine? I think this is called isopathy or homeoprophylaxis. The point is, these statements are not a definition for what constitutes a vaccine.
They state “protection” but what is doing the protecting? I notice they don’t mention the production of antibodies. Maybe because they know the type of antibodies produced don’t confer long term immunity. But that’s a whole other rabbit hole looking into vaccination versus immunisation!
How does the FDA regulate “vaccines”?
In the United States the Federal Drug Administration (FDA) has a separate department called the Center for Biologics Evaluation and Research (CBER) which is responsible for assessing all “therapeutic biological products”, of which vaccines are included. I recall some time ago when Robert F. Kennedy Jr. mentioned that vaccines go through a different regulatory assessment process than drug medicines, a more lax regulatory pathway that has been grandfathered in from war time. If our TGA defer to the FDA’s assessment, then vaccine assessment process is not “extremely rigorous, comprehensive [or] independent”4 as they’d have you believe. Put a pin in this information.56
(Today’s CBER has a long history dating back to the Biologics Control Act of 1902 because products deemed to be “vaccines” or “antitoxins” were killing children in “therapeutic disasters”!78 )
CBER defined a “biologic” as:
Biologics, in contrast to drugs that are chemically synthesized, are derived from living sources (such as humans, animals, and microorganisms). Most biologics are complex mixtures that are not easily identified or characterized, and many biologics are manufactured using biotechnology.
or put more simply:
Biologics are medical products derived from living sources.
or Health Canada define Vaccines as Biologics because of their variable “biological nature”!
I wonder, can a manufacturer/sponsor, simply submit their product dossier for regulatory consideration, and refer to it as a “vaccine”. Would the TGA Secretary just accepts this classification as fact and thus move it into the “vaccine” regulatory pathway? The point is, based on what criteria does the TGA admit a product into the vaccine regulatory pathway?
Gene Technology Regulator
The reason why I’m focusing on the TGA, is because Australia’s “gene technology regulator” did not have any oversight of the genetically modified RNA products by Moderna and Pfizer-BioNTech, all regulatory oversight was deferred to the TGA who, the TGA secretary, determined in 2020, that these gene therapy products could enter the Provisional Registration regulatory pathway under the classification of “vaccine”.
If the Office of Gene Technology Regulator (OGTR) is able to determine that the only “gene technology” product that comes under their purview are “organisms”, then surely the TGA should have a publicly available definition for a “vaccine”.
For the COVID-19 vaccine products, the TGA took on 100% responsibility of being expert in assessing a, never-used-before, brand new genetic technology product. Did the Advisory Committee on Vaccines actually have the necessary expertise for assessing such technology?
I keep thinking that the TGA actually deferred to the FDA or maybe one of their ACCESS Consortium regulatory partners for some alleged expertise?
When you consider the FDA charges over $2 million to assess a new clinical trial product, versus the seemingly smaller fees charged by the TGA, it makes me think the TGA does outsource their decisions!
The Regulations set the cost schedule for which they TGA states:
The TGA is required to recover its costs for all activities that fall within the scope of the Therapeutic Goods Act 1989, including the TGA's public health responsibilities
As I was working through this process I was unsure whether vaccines would be considered under Prescription or non-prescription medicine? Since vaccines can be given out in a pharmacy without a prescription, they could be considered an over-the-counter medicine! But no…
It would appear that vaccines are considered a prescription medicine, but are evaluated as an “other” category. It’s all very confusing.
Prescription medicines and other medicines that are evaluated as prescription medicines.
When Biologicals are not Biologicals
The TGA legislation refers in many places to Biologicals, that being specifically human cell and blood products, but there is also reference to Biologicals in relation to Medicines, this makes reading the Regulations very confusing, as one is legally separate from the other.
There is a category of Biological Medicines, which I touched on earlier, and where I assume vaccines fall.
Look at the January 2019 version of The Act, at “Part 3‑2A—Biologicals” then “32A Meaning of biological”. This had me confused from a long time, it appears strictly to be for human derived products, and not for pathogens, but in reading this it appears that the TGA Secretary has all the power in determining whether a product will be accepted or rejected as a “biological” that can be used “in the treatment or prevention of a disease”. When “prevention of a disease” is stated, I think they could use this for vaccines.
It appears that the TGA Secretary has all the medical regulatory power.
The Secretary of the TGA is officially known as the Deputy Secretary of the Health Products Regulation Group of the Australian Department of Health and Aged Care, was held by Adjunct Professor John Skerritt from 2012- 2023. During the COVID-19 pandemic, he was the one person that “determined” which COVID-19 vaccines could enter the fast tracked regulatory pathway under the emergency “Provisional Registration” process. He possessed all the power. Skerritt retired April 2023, and was replaced by Tasmanian Prof Tony Lawler.
Skerritt now resides on the board of lobby groups Medicines Australia9 and AusBiotech10 . Revolving door anyone?
So I still don’t have a legal definition of a “vaccine”.
https://web.archive.org/web/20190227224025/https://www.tga.gov.au/tga-regulatory-framework
https://www.tga.gov.au/how-we-regulate
TGA: Who we are & what we do (2019 version)
https://web.archive.org/web/20190130052418/http://www.tga.gov.au/who-we-are-what-we-do
https://web.archive.org/web/20221118065723/https://www.health.gov.au/news/tga-to-give-australians-confidence-to-get-the-covid-19-jab
https://web.archive.org/web/20010915013943/http://www.fda.gov/cber/about.htm
https://www.fda.gov/about-fda/fda-organization/center-biologics-evaluation-and-research-cber
https://web.archive.org/web/20090119132120/http://www.fda.gov/cber/inside/centbiolcont.htm
https://web.archive.org/web/20090119132122/http://www.fda.gov/fdac/features/2002/402_bio.html
https://www.medicinesaustralia.com.au/media-release/professor-john-skerritt-appointed-to-medicines-australia-board/
https://www.ausbiotech.org/about-us/biographies/professor-john-skerritt












Unfortunately for a rationally thinking honest Australian citizen it is currently futile to try to honestly deal with a corrupt government agency as the TGA.
Great research.
Thanks & I will share.